-
PDF
- Split View
-
Views
-
Cite
Cite
Leixi Xue, Zhiqin Liu, Ji Hu, Jun Huang, Jian Wen, Zhichun Liu, Estrogen-induced expression of tumor necrosis factor-like weak inducer of apoptosis through ERα accelerates the progression of lupus nephritis, Rheumatology, Volume 55, Issue 10, October 2016, Pages 1880–1888, https://doi.org/10.1093/rheumatology/kew248
Close - Share Icon Share
Abstract
Objectives. Oestrogens have been shown to play key roles in the pathogenesis of SLE. The aim of this study was to investigate the roles and mechanisms of 17β-estradiol (E2) in TNF-like weak inducer of apoptosis (TWEAK) expression in LN.
Methods. Peripheral blood mononuclear cells (PBMCs) obtained from LN patients were used for in vitro experiments, while female MRL/lpr and MRL/MpJ mice were used for in vivo studies. E2, ICI 182 780 [estrogen receptor (ER)-selective antagonist], methyl-piperidino-pyrazole (MPP, ERα-selective modulator), lentivirus (LV)-TWEAK–short hairpin RNA (shRNA) and LV-control-shRNA treatments were used in this study.
Results. TWEAK mRNA expression in PBMCs was significantly increased following E2 treatment and downregulated after incubation with ICI 182 780 or MPP. Compared with sham-operated MRL/lpr mice, ovariectomized mice, treated with dimethyl sulphoxide vehicle alone, showed lower expression levels of renal TWEAK mRNA and protein. The expression of both mRNA and protein in ovariectomized mice was upregulated after E2 treatment and downregulated after ICI 182 780 or MPP co-treatment. Severe renal damage was observed in E2-treated ovariectomized mice, as were higher serum levels of IL-6, compared with dimethyl sulphoxide vehicle–treated ovariectomized mice. Co-treatment with LV-TWEAK-shRNA reversed these changes, and LV-control-shRNA treatment had no effect on them.
Conclusion. Our results demonstrated that E2 plays an important role in the upregulation of TWEAK expression in LN, most likely through an ERα-dependent pathway, causing kidney damage. This provides a novel insight into the mechanisms of the E2-TWEAK signalling pathway in LN.
Rheumatology key messages
17β-estradiol induces tumor necrosis factor-like weak inducer of apoptosis expression through ERα in lupus nephritis.
Tumor necrosis factor-like weak inducer of apoptosis upregulates serum interleukin-6 expression in lupus mice.
Gene silence of tumor necrosis factor-like weak inducer of apoptosis plays a protective role in lupus nephritis.
Introduction
TNF-like weak inducer of apoptosis (TWEAK) is a member of the TNF ligand superfamily [1]. As a pro-inflammatory cytokine, TWEAK has been shown to play important roles in SLE, especially in LN [2–6]. Renal TWEAK expression and urinary TWEAK (uTWEAK) levels are significantly higher in SLE patients with renal involvement than in patients without the renal involvement [2–6]. Although TWEAK regulates the inflammatory reactions characterized by an increase in the levels of many cytokines (such as IL-6, IL-10, and monocyte chemoattractant protein-1), leading to renal injury in SLE [2–6], its upstream regulatory mechanisms remain unclear.
Cross-talk between endocrine- disrupting chemicals and cytokines plays a very important role in the pathogenesis of autoimmune diseases. The estrogen receptor (ER)/cytokine signalling pathway is one of the examples of endocrine–immune system interplay in SLE. It was previously reported that serum levels of soluble TWEAK (sTWEAK) in female patients with acute ST-elevation myocardial infarction (MI) were significantly higher compared with male patients or patients with stable chronic coronary artery disease [7]. Furthermore, female NZB.Yaa mice have significantly higher levels of renal TWEAK mRNA expression compared with male NZB mice [8–10]. The expression of TWEAK mRNA in endometrial samples was shown to be significantly different between the proliferative phase and the secretory phase, suggesting that female sex hormones may affect TWEAK expression in the endometrium [11, 12]. TWEAK expression in endometrial stromal cells was positively correlated with the concentration of oestrogen [11]. These studies suggest that the increase in TWEAK expression may be partly due to female sex hormones.
SLE shows a strong gender bias and occurs ten times more often in females than in males [13]. SLE patients experience an increase in lupus flares during pregnancy [14, 15], which may be due to different steroid hormone and cytokine profiles in SLE patients compared with healthy subjects, leading to a dysregulation of the balance between cell-mediated and humoral immune response [16–18]. In many genetic-based murine models of SLE, including MRL/lpr and NZB/NZW F1 mice, females develop more severe disease and higher titres of autoantibodies at an earlier age, compared with males [19–21]. Circulating oestrogens exert their effects by binding to their intracellular receptors [22], ER alpha (ERα) and beta (ERβ) isoforms, and ERα was shown to play a key role in modulation of lupus [23–26]. Administration of 17β-estradiol (E2) to male and female castrated MRL/lpr mice accelerates lymphoproliferation, glomerulonephritis and mortality [21].
The MRL/lpr mouse is a commonly used animal model that shows pathologies resembling human SLE, including significant numbers of serum autoantibodies, together with immune complex glomerulonephritis and vasculitis [27]. Based on the previous studies, we hypothesized that TWEAK is potentially regulated by E2 through ERα in LN. Here, we examined how E2 regulates TWEAK signalling in peripheral blood mononuclear cells (PBMCs) and in the MRL/lpr mouse model, in order to improve our understanding of its role in LN.
Materials and methods
Patients
The study protocol and all experimental procedures were approved by the Human Ethics Review Committee of the Second Affiliated Hospital of Soochow University, Suzhou, China. Twelve female patients with LN, 14–48 years old [mean 36.8 (9.2) years] who were treated in our department (Department of Rheumatology and Immunology, the Second Affiliated Hospital of Soochow University) between 2013 and 2014 were included in this study, according to the SLE and LN classification criteria of ACR (supplementary Table 1, available at Rheumatology Online) [28]. The 12 patients signed an informed consent form approved by the Human Ethics Review Committee of the Second Affiliated Hospital of Soochow University. None of the patients received immunomodulatory therapy during the last month prior to blood collection.
Isolation and culture of peripheral blood mononuclear cells
Isolation of PBMCs was carried out using Ficoll (GE Healthcare, Uppsala, Sweden), according to the manufacturer’s instructions. Purified human PBMCs were cultured in phenol red–free Roswell Park Memorial Institute (Life Technologies, Grand Island, NY, USA) tissue culture medium containing 5% charcoal-stripped fetal bovine serum (Life Technologies, Grand Island, NY, USA). Afterward, they were divided equally into four groups and treated for 24 h with one of the following: 1 × 10−5 mmol/l E2 (Sigma Aldrich, St Louis, MO, USA), 1 × 10−5 mmol/l E2 combined with 1 × 10−4 mmol/l ICI 182 780 (ER-selective antagonist) (Santa Cruz, CA, USA), 1 × 10−5 mmol/l E2 with 1 × 10−2 mmol/l methyl-piperidino-pyrazole (MPP, ERα-selective modulator) (Tocris Cookson, Inc., Ellisville, MO, USA) or dimethyl sulphoxide (DMSO) vehicle alone [29].
Mouse models
Female MRL/lpr and MRL/MpJ mice (Shanghai Slack Laboratory Animal Co. Ltd, Shanghai, China) were bred and maintained in the animal facility of the Second Affiliated Hospital of Soochow University. All animal study protocols were approved by the Institutional Animal Care and Use Committee of the Second Affiliated Hospital of Soochow University, Suzhou, China. Ovariectomy was performed on 12-week-old MRL/lpr female mice [30]. One week later, they were intraperitoneally injected twice per week with one of the following treatments: DMSO vehicle alone; 5 μg E2; 5 μg E2 combined with 50 μg ICI 182 780; 5 μg E2 with 50 μg MPP. The applied doses were chosen based on the previous studies that investigated the effects of β-estradiol and ER-selective antagonists in mice [29–32]. A different group of ovariectomized MRL/lpr female mice was administered, through the tail vein, either 2 × 107 transducing units of lentivirus (LV)-TWEAK-short hairpin RNA (TWEAK-shRNA group) or 2 × 107 transducing units LV-control-shRNA (control-shRNA group). The same surgical procedure was performed on the sham-operated control animals, but the ovaries were not removed. The mice were sacrificed at days 8, 15 and 29 after the treatment.
The shRNAs targeting mouse TWEAK gene (GenBank accession number: NM_011614) were designed using siRNA Target Finder and Design Tool (available at: http://www.ambion.com), and were commercially obtained from GeneChem (Shanghai, China). The sequences of LV-TWEAK-shRNA were (forward) 5′-CGG TAA CCT ACT TTG GAC TCT TTC CTC GAG GAA AGA GTC CAA AGT AGG TTA TTT TTG-3′ and (reverse) 5′-AAT TCA AAA AAA TAC TTT GGA CTC TTT CCT CGA GGA AAG AGT CCA AAG TAG GTT A-3′, and the sequences of LV-control-shRNA were (forward) 5′-CCG GTT CTC CGA ACG TGT CAC GTT TCA AGA GAA CGT GAC ACG TTC GGA GAA TTT TTG-3′, and (reverse) 5′-AAT TCA AAA ATT CTC CGA ACG TGT CAC GTT CTC TTG AAA CGT GAC ACG TTC GGA GAA-3′.
Quantitative real-time PCR
Total RNA was extracted from PBMCs or kidney tissue using Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Reverse transcription was performed with 3 μg of total RNA using the M-MuLV First Strand cDNA Synthesis Kit from Sangon Biotech (Shanghai, China). Quantitative real-time PCR was performed in triplicate using the SYBR green stain and the CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA) under the following conditions: 10 min at 95 °C, and 45 cycles of 95 °C for 10 s, 60 °C for 20 s and 72 °C for 30 s. The primers used for mouse TWEAK amplification were: (forward) 5′-CCA TCC CAC CAC AAC TAT CC-3′ and (reverse) 5′-AGG TCC AGC CCA TCT CAG T-3′ (126 bps); mouse β-actin primer sequences were: (forward) 5′-GAG ACC TTC AAC ACC CCA GC-3′ and (reverse) 5′-ATG TCA CGC ACG ATT TCC C-3′ (263 bps); human TWEAK primer sequences were 5′-GAG GAA GCC AGA TCA ACA G-3′ and (reverse) 5′-CAC CAT CCA CCA GCA AGT C-3′ (157 bps); human β-actin primers were: (forward) 5′-TGA CGT GGA CAT CCG CAA AG-3′ and (reverse) 5′-CTG GAA GGT GGA CAG CGA GG-3′ (205 bps). Quantitative real-time PCR results were normalized to β-actin expression levels, and all enhancements or repressions of TWEAK expression were analysed as relative fold changes tentatively assigned +1. Relative gene expression changes were calculated as fold differences using the 2−ΔΔCt method.
Western blotting
Total cell lysates from kidney tissue samples were collected in PBS and resuspended in a modified radio-immune precipitation assay lysis buffer (50 mM Tris-HCl, pH 8.0, 250 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS), supplemented with protease inhibitor (Roche Diagnostics, Mannheim, Germany) and incubated at 4 °C for 30 min. Cell lysates were centrifuged at 14 000 rpm in a microcentrifuge for 10 min at 4°C. Supernatants were collected, and the protein concentration was measured using the Bio-Rad protein assay kit (Beyotime Institute of Biotechnology, Shanghai, China). Total proteins (60 μg) were used for western blotting. Goat antibodies specific for TWEAK (1:500, Santa Cruz, USA) and rabbit polyclonal anti-β-actin antibody (Sangon Biotech, Shanghai, China) were used. Quantity One analysis software (Bio-Rad Laboratories) was used to perform density analysis. TWEAK protein expression was quantified by the FluorChem FC2 system (NatureGene Corpration, NJ, USA). All data are expressed as the ratio of TWEAK integral optical density and β-actin integral optical density in the same sample.
Histopathological analysis
The kidneys were dissected, fixed in 10% buffered formalin, and paraffin-embedded sections of kidney tissues (3-mm-thick) were stained with haematoxylin and eosin (H&E), periodic acid-Schiff (PAS) and Masson’s trichrome staining for histopathological examinations at day 29. The sections were evaluated blindly by an experienced pathologist, as previously described [33, 34]. The presence of glomerulonephritis was evaluated by light microscopy. PAS-positive deposits were each scored from zero to four (0, absent; 1, mild; 2, mild–moderate; 3, moderate; 4, severe). The presence of inflammatory cell infiltrates, observed by H&E staining, and the aniline blue–positive areas (fibrotic areas), observed by Masson’s trichrome staining, were each graded on a scale from zero to four (0, absent; 1, present in <25% of the section; 2, present in 25–50% of the section; 3, present in 50–75% of the section; and 4, present in >75% of the section).
24-h urinary albumin, serum creatinine and blood urea nitrogen screening
Overnight urine samples were collected by metabolism cages, and albumin levels were monitored using Coomassie Brilliant Blue G-250 (Beyotime Institute of Biotechnology, Shanghai, China). Serum creatinine and blood urea nitrogen levels were determined by colorimetric analysis kits (Gotofcm Biotech, Hangzhou, China).
ELISA
Anti-dsDNA (anti-dsDNA) IgG (Biorui Biotech, Shanghai, China) and IL-6 (Gotofcm Biotech, Hangzhou, China) levels were measured using sandwich ELISA kits, according to the manufacturer’s instructions.
Statistical analysis
All data were expressed as mean (s.d.). A one-way analysis of variance test was used for comparison of more than two groups. The differences between the groups were assessed with the Bonferroni post hoc test. The datasets were analysed using the SPSS version 13.0 statistical package. Each experiment was repeated at least three times, to ensure reproducibility. P-values of ⩽ 0.05 were considered statistically significant.
Results
E2 upregulated TWEAK mRNA expression through ERα in PBMCs obtained from LN patients
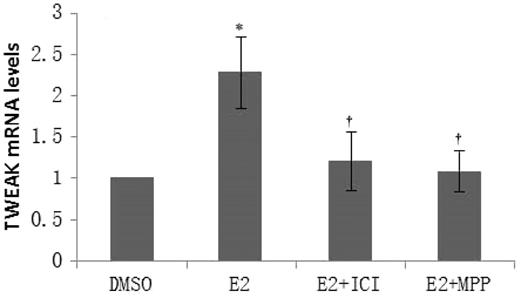
We have previously shown that TWEAK expression in PBMCs obtained from SLE patients was increased compared with PBMCs from healthy controls, and that it was positively correlated with renal damage [5]. In this study, we found that TWEAK mRNA levels, in PBMCs obtained from LN patients, which were cultured with E2, were significantly higher [2.40 (0.37)] compared with the levels in PBMCs cultured with DMSO vehicle alone (indicated as 1, P < 0.05), and this upregulation was reversed by co-culture with ICI 182 780 [1.21 (0.35), P < 0.05] or MPP [1.09 (0.24), P < 0.05] (Fig. 1).
17β-estradiol upregulates TNF-like weak inducer of apoptosis mRNA expression through estrogen receptor alpha (ERα) in peripheral blood mononuclear cells obtained from LN patients
Peripheral blood mononuclear cells (PBMCs) from LN patients were treated with DMSO vehicle, 17β-estradiol (E2), E2 + ICI 182 780, and 17β-estradiol (E2) + methyl-piperidino-pyrazole (MPP) for 24 h. Total RNA from PBMCs was extracted for quantitative real-time PCR. The ratio of TNF-like weak inducer of apoptosis (TWEAK)/β-actin mRNA levels was calculated. The ratio of mRNA levels in cells treated with dimethyl sulphoxide (DMSO) vehicle was designated as one. The error bars represent standard deviations. DMSO, PBMCs treated with DMSO alone; E2, PBMCs treated with E2; E2 + ICI, PBMCs treated with E2 + ICI 182 780; E2 + MPP, PBMCs treated with E2 + MPP. *P < 0.05 compared with TWEAK mRNA levels of PBMCs treated with DMSO alone, †P < 0.05 compared with TWEAK mRNA levels of PBMCs treated with E2.
E2 increased TWEAK mRNA and protein levels through ERα in MRL/lpr mice
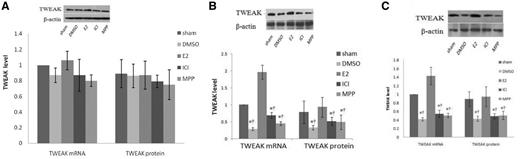
Quantitative real-time PCR results showed no significant differences in TWEAK mRNA levels between the investigated groups at day 8 (Fig. 2A), whereas at day 15 (Fig. 2B), TWEAK mRNA levels in ovariectomized MRL/lpr mice, which were administrated the DMSO vehicle alone, were significantly lower than in sham-operated mice (P < 0.05). E2 treatment increased TWEAK mRNA expression about 6- to 7-fold (P < 0.05), while co-treatment with ICI 182 780 or MPP downregulated its expression to ∼35% (P < 0.05) and 23% (P < 0.05), respectively. Similar results were obtained at day 29 (Fig. 2C).
17β-estradiol increases TNF-like weak inducer of apoptosis expression in MRL/lpr mice through estrogen receptor alpha (ERα)
Total kidney lysates were collected for quantitative real-time PCR (left bottom) and western blotting (top and right bottom), in order to measure TNF-like weak inducer of apoptosis (TWEAK) expression at days 8 (A), 15 (B) and 29 (C). The ratio of TWEAK/β-actin mRNA levels in sham-operated MRL/lpr mice was designated as one. The error bars represent standard deviations. Sham, MRL/lpr female mice with sham operation; DMSO, MRL/lpr female mice with ovariectomy treated with dimethyl sulphoxide (DMSO) vehicle alone; E2, MRL/lpr female mice with ovariectomy treated with 17β-estradiol (E2); ICI, MRL/lpr female mice with ovariectomy treated with E2 plus ICI 182 780; MPP, MRL/lpr female mice with ovariectomy treated with E2 plus methyl-piperidino-pyrazole (MPP). *P < 0.05 compared with ovariectomized mice treated with E2, †P < 0.05 compared with sham-operated mice.
The TWEAK protein levels detected by western blotting were consistent with the TWEAK mRNA levels obtained using quantitative real-time PCR.
LV-TWEAK-shRNA treatment decreased TWEAK mRNA expression in MRL/lpr female mice
No significant differences were found in TWEAK mRNA levels between the groups at day 8 (Fig. 3A), whereas at days 15 (Fig. 3B) and 29 (Fig. 3C), LV-TWEAK-shRNA treatment reduced TWEAK mRNA expression induced by E2 to ∼20% (P < 0.05) and ∼33% (P < 0.05), respectively. LV-control-shRNA treatment showed no effect on TWEAK mRNA expression.
LV-TWEAK-shRNA treatment inhibits TNF-like weak inducer of apoptosis mRNA expression in MRL/lpr female mice
Total RNA was extracted from kidneys and subjected to quantitative real-time PCR analysis, in order to analyse TNF-like weak inducer of apoptosis (TWEAK) mRNA levels at Days 8 (A), 15 (B) and 29 (C). The ratio of TWEAK/β-actin mRNA levels in sham-operated MRL/lpr mice was designated as one. The error bars represent standard deviations. Sham, MRL/lpr female mice with sham operation; DMSO, MRL/lpr female mice with ovariectomy treated with dimethyl sulphoxide (DMSO) vehicle alone; E2, MRL/lpr female mice with ovariectomy treated with 17β-estradiol (E2); TWEAK-shRNA, MRL/lpr female mice with ovariectomy treated with E2 plus lentivirus-TWEAK–short hairpin RNA (LV-TWEAK-shRNA); control-shRNA, MRL/lpr female mice with ovariectomy treated with E2 plus LV-control-shRNA. *P < 0.05 compared with ovariectomized mice treated with E2, †P < 0.05 compared with sham-operated mice.
Treatment with LV-TWEAK-shRNA decreased renal damage aggravated by E2 in MRL/lpr mice
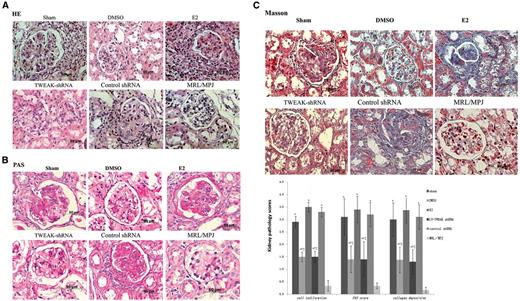
We examined histological changes in the MRL/lpr mice and MRL/MpJ mice on treatment day 29. Compared with MRL/MpJ mice, in sham-operated MRL/lpr mice histological evidence of severe glomerulonephritis was observed, characterized by glomerular hypercellularity, PAS-positive material and collagenous fibre deposition (P < 0.05). Compared with sham-operated MRL/lpr mice, ovariectomized mice treated with DMSO showed that glomerulonephritis was significantly alleviated, while E2 treatment aggravated the pathological changes in the kidneys (P < 0.05). Co-treatment with LV-TWEAK-shRNA but not LV-control-shRNA alleviated E2-induced glomerulonephritis (P < 0.05) (Fig. 4).
Histopathological changes in MRL/lpr mouse kidneys at day 29
(A) Representative haematoxylin and eosin (H&E) sections of formalin-fixed kidneys; (B) representative micrographs of periodic acid-Schiff (PAS)-stained sections from paraffin-embedded tissue; (C) representative micrographs of Masson-stained sections of paraffin-embedded tissue. The kidney pathological scores of cell infiltration, PAS-positive material, and collagenous fibre deposition are shown at the bottom. The error bars represent standard deviations. Sham, MRL/lpr female mice with sham operation; DMSO, MRL/lpr female mice with ovariectomy treated with dimethyl sulphoxide (DMSO) vehicle alone; E2, MRL/lpr female mice with ovariectomy treated with 17β-estradiol (E2); TWEAK-shRNA, MRL/lpr female mice with ovariectomy treated with E2 plus lentivirus-TNF-like weak inducer of apoptosis–short hairpin RNA (LV-TWEAK-shRNA); control-shRNA, MRL/lpr female mice with ovariectomy treated with E2 plus LV-control-shRNA. *P < 0.05 compared with MRL/MPJ female mice, †P < 0.05 compared with ovariectomized MRL/lpr female mice treated with E2, ‡P < 0.05 compared with sham-operated MRL/lpr female mice. Scale bar 50 μm.
We also determined the levels of 24-h urinary albumin, serum creatinine, and blood urea nitrogen at day 29 (Table 1). The sham-operated MRL/lpr mice developed severe albuminuria [7.80 (1.21) mg] compared with MRL/MpJ mice [1.03 (0.24) mg] (P < 0.05), but the 24-h urinary albumin levels decreased to 4.19 (0.54) mg following ovariectomy (P < 0.05). As expected, E2 treatment significantly increased the levels of urinary albumin [8.17 (1.02) mg], which was downregulated by co-treatment with LV-TWEAK-shRNA [5.23 (0.49) mg] (P < 0.05). Urinary albumin levels did not differ following the LV-control-shRNA co-treatment [7.67 (1.17) mg].
The index levels of renal damage in different groups of MRL/lpr mice at day 29
| Group . | 24-h urinary albumin (mg) . | Scr (mmol/l) . | BUN (μmol/l) . |
|---|---|---|---|
| Sham | 7.80 (1.2)* | 7.94 (1.03)* | 98.63 (10.05)* |
| DMSO | 4.19 (0.54)*,†,‡ | 5.76 (1.10)*,†,‡ | 57.80 (4.89)*,†,‡ |
| E2 | 8.17 (1.02)* | 8.24 (1.04)* | 94.52 (9.27)* |
| TWEAK- shRNA | 5.23 (0.49)*,†,‡ | 6.11 (1.20)*,†,‡ | 65.69 (3.95)*,†,‡ |
| Control-shRNA | 7.67 (1.17)* | 7.90 (1.10)* | 100.31 (11.4)* |
| MRL/MPJ | 1.03 (0.24) | 5.01 (0.84) | 38.73 (4.64) |
| Group . | 24-h urinary albumin (mg) . | Scr (mmol/l) . | BUN (μmol/l) . |
|---|---|---|---|
| Sham | 7.80 (1.2)* | 7.94 (1.03)* | 98.63 (10.05)* |
| DMSO | 4.19 (0.54)*,†,‡ | 5.76 (1.10)*,†,‡ | 57.80 (4.89)*,†,‡ |
| E2 | 8.17 (1.02)* | 8.24 (1.04)* | 94.52 (9.27)* |
| TWEAK- shRNA | 5.23 (0.49)*,†,‡ | 6.11 (1.20)*,†,‡ | 65.69 (3.95)*,†,‡ |
| Control-shRNA | 7.67 (1.17)* | 7.90 (1.10)* | 100.31 (11.4)* |
| MRL/MPJ | 1.03 (0.24) | 5.01 (0.84) | 38.73 (4.64) |
Data are presented as mean (s.d.).
*P < 0.05 compared with MRL/MpJ female mice.
†P < 0.05 compared with ovariectomized MRL/lpr female mice treated with 17β-estradiol (E2).
‡P < 0.05 compared with sham-operated MRL/lpr female mice. Scr: serum creatinine; BUN: blood urea nitrogen; Sham: MRL/lpr female mice with sham operation; DMSO: MRL/lpr female mice with ovariectomy treated with dimethyl sulphoxide (DMSO) vehicle alone; E2: MRL/lpr female mice with ovariectomy treated with E2; TWEAK-shRNA: MRL/lpr female mice with ovariectomy treated with E2 plus lentivirus-TNF-like weak inducer of apoptosis–short hairpin RNA (LV-TWEAK-shRNA); control-shRNA: MRL/lpr female mice with ovariectomy treated with E2 plus LV-control-shRNA.
The index levels of renal damage in different groups of MRL/lpr mice at day 29
| Group . | 24-h urinary albumin (mg) . | Scr (mmol/l) . | BUN (μmol/l) . |
|---|---|---|---|
| Sham | 7.80 (1.2)* | 7.94 (1.03)* | 98.63 (10.05)* |
| DMSO | 4.19 (0.54)*,†,‡ | 5.76 (1.10)*,†,‡ | 57.80 (4.89)*,†,‡ |
| E2 | 8.17 (1.02)* | 8.24 (1.04)* | 94.52 (9.27)* |
| TWEAK- shRNA | 5.23 (0.49)*,†,‡ | 6.11 (1.20)*,†,‡ | 65.69 (3.95)*,†,‡ |
| Control-shRNA | 7.67 (1.17)* | 7.90 (1.10)* | 100.31 (11.4)* |
| MRL/MPJ | 1.03 (0.24) | 5.01 (0.84) | 38.73 (4.64) |
| Group . | 24-h urinary albumin (mg) . | Scr (mmol/l) . | BUN (μmol/l) . |
|---|---|---|---|
| Sham | 7.80 (1.2)* | 7.94 (1.03)* | 98.63 (10.05)* |
| DMSO | 4.19 (0.54)*,†,‡ | 5.76 (1.10)*,†,‡ | 57.80 (4.89)*,†,‡ |
| E2 | 8.17 (1.02)* | 8.24 (1.04)* | 94.52 (9.27)* |
| TWEAK- shRNA | 5.23 (0.49)*,†,‡ | 6.11 (1.20)*,†,‡ | 65.69 (3.95)*,†,‡ |
| Control-shRNA | 7.67 (1.17)* | 7.90 (1.10)* | 100.31 (11.4)* |
| MRL/MPJ | 1.03 (0.24) | 5.01 (0.84) | 38.73 (4.64) |
Data are presented as mean (s.d.).
*P < 0.05 compared with MRL/MpJ female mice.
†P < 0.05 compared with ovariectomized MRL/lpr female mice treated with 17β-estradiol (E2).
‡P < 0.05 compared with sham-operated MRL/lpr female mice. Scr: serum creatinine; BUN: blood urea nitrogen; Sham: MRL/lpr female mice with sham operation; DMSO: MRL/lpr female mice with ovariectomy treated with dimethyl sulphoxide (DMSO) vehicle alone; E2: MRL/lpr female mice with ovariectomy treated with E2; TWEAK-shRNA: MRL/lpr female mice with ovariectomy treated with E2 plus lentivirus-TNF-like weak inducer of apoptosis–short hairpin RNA (LV-TWEAK-shRNA); control-shRNA: MRL/lpr female mice with ovariectomy treated with E2 plus LV-control-shRNA.
The serum creatinine and blood urea nitrogen levels showed similar changes to those observed in the 24-h urinary albumin levels for the various groups (Table 1). Serum levels of creatinine [8.24 (1.04) mmol/l] and blood urea nitrogen [94.52 (9.27) μmol/l] in E2-treated ovariectomized mice were significantly higher than those in DMSO-treated ovariectomized mice [5.76 (1.10) mmol/l and 57.80 (4.89) μmol/l, respectively] (both P < 0.05). After co-treatment with LV-TWEAK-shRNA, the levels of serum creatinine and blood urea nitrogen decreased to 6.11 (1.20) mmol/l and 65.69 (3.95) μmol/l, respectively (both P < 0.05). LV-control-shRNA treatment had no effect on the levels of serum creatinine or blood urea nitrogen.
LV-TWEAK-shRNA treatment downregulated the levels of serum anti-dsDNA IgG and IL-6 induced by E2 in MRL/lpr mice
We evaluated the serum levels of anti-dsDNA IgG and IL-6, using ELISA. At day 8 (Table 2), there were no differences in the serum IL-6 levels between groups. Ovariectomized female mice treated with DMSO vehicle had significantly lower serum IL-6 levels [53.5 (5.1) pg/ml] compared with sham-operated mice [116.8 (19.8) pg/ml, P < 0.05] at day 15 (Table 2). E2 treatment led to the upregulation of the serum IL-6 levels in ovariectomized mice [120.3 (16.3) pg/ml, P < 0.05], and this upregulation was inhibited by co-treatment with LV-TWEAK-shRNA [33.1 (10.1) pg/ml, P < 0.05], but not with LV-control-shRNA [113.9 (12.9) pg/ml]. Similar results were obtained at day 29 (Table 2).
The serum levels of IL-6 and anti-dsDNA IgG in different MRL/lpr mice
| Group . | IL-6 . | anti-dsDNA IgG . | ||||
|---|---|---|---|---|---|---|
| . | Day 8 . | Day 15 . | Day 29 . | Day 8 . | Day 15 . | Day 29 . |
| Sham | 128.6 (16.6) | 116.8 (19.8) | 184.8 (12.9) | 246.1 (16.3) | 307.1 (12.6) | 313.8 (19.9) |
| DMSO | 114.2 (16.7) | 53.5 (5.1)† | 56.7 (17.0)† | 231.4 (22.4) | 144.1 (17.1)† | 162.7 (8.6)† |
| E2 | 146.7 (14.6) | 120.3 (16.3) | 180.5 (5.7) | 266.5 (11.4) | 292.8 (20.6) | 305.3 (27.7) |
| TWEAK- shRNA | 115.3 (10.4) | 33.1 (10.1)* | 48.1 (21.0)* | 235.5 (22.1) | 144.4 (35.0)* | 275.9 (37.5) |
| Control-shRNA | 134.9 (16.8) | 113.9 (12.9) | 172.5 (16.6) | 256.7 (18.4) | 294.6 (40.5) | 298.4 (17.8) |
| Group . | IL-6 . | anti-dsDNA IgG . | ||||
|---|---|---|---|---|---|---|
| . | Day 8 . | Day 15 . | Day 29 . | Day 8 . | Day 15 . | Day 29 . |
| Sham | 128.6 (16.6) | 116.8 (19.8) | 184.8 (12.9) | 246.1 (16.3) | 307.1 (12.6) | 313.8 (19.9) |
| DMSO | 114.2 (16.7) | 53.5 (5.1)† | 56.7 (17.0)† | 231.4 (22.4) | 144.1 (17.1)† | 162.7 (8.6)† |
| E2 | 146.7 (14.6) | 120.3 (16.3) | 180.5 (5.7) | 266.5 (11.4) | 292.8 (20.6) | 305.3 (27.7) |
| TWEAK- shRNA | 115.3 (10.4) | 33.1 (10.1)* | 48.1 (21.0)* | 235.5 (22.1) | 144.4 (35.0)* | 275.9 (37.5) |
| Control-shRNA | 134.9 (16.8) | 113.9 (12.9) | 172.5 (16.6) | 256.7 (18.4) | 294.6 (40.5) | 298.4 (17.8) |
Data are presented as mean (s.d.).
*P < 0.05 compared with ovariectomized MRL/lpr female mice treated with 17β-estradiol (E2) at the same time.
†P < 0.05 compared with sham-operated MRL/lpr female mice at the same time. Sham: MRL/lpr female mice with sham operation; DMSO: MRL/lpr female mice with ovariectomy treated with dimethyl sulphoxide (DMSO) vehicle alone; E2: MRL/lpr female mice with ovariectomy treated with E2; TWEAK-shRNA: MRL/lpr female mice with ovariectomy treated with E2 plus lentivirus-TNF-like weak inducer of apoptosis–short hairpin RNA (LV-TWEAK-shRNA); control-shRNA: MRL/lpr female mice with ovariectomy treated with E2 plus LV-control-shRNA.
The serum levels of IL-6 and anti-dsDNA IgG in different MRL/lpr mice
| Group . | IL-6 . | anti-dsDNA IgG . | ||||
|---|---|---|---|---|---|---|
| . | Day 8 . | Day 15 . | Day 29 . | Day 8 . | Day 15 . | Day 29 . |
| Sham | 128.6 (16.6) | 116.8 (19.8) | 184.8 (12.9) | 246.1 (16.3) | 307.1 (12.6) | 313.8 (19.9) |
| DMSO | 114.2 (16.7) | 53.5 (5.1)† | 56.7 (17.0)† | 231.4 (22.4) | 144.1 (17.1)† | 162.7 (8.6)† |
| E2 | 146.7 (14.6) | 120.3 (16.3) | 180.5 (5.7) | 266.5 (11.4) | 292.8 (20.6) | 305.3 (27.7) |
| TWEAK- shRNA | 115.3 (10.4) | 33.1 (10.1)* | 48.1 (21.0)* | 235.5 (22.1) | 144.4 (35.0)* | 275.9 (37.5) |
| Control-shRNA | 134.9 (16.8) | 113.9 (12.9) | 172.5 (16.6) | 256.7 (18.4) | 294.6 (40.5) | 298.4 (17.8) |
| Group . | IL-6 . | anti-dsDNA IgG . | ||||
|---|---|---|---|---|---|---|
| . | Day 8 . | Day 15 . | Day 29 . | Day 8 . | Day 15 . | Day 29 . |
| Sham | 128.6 (16.6) | 116.8 (19.8) | 184.8 (12.9) | 246.1 (16.3) | 307.1 (12.6) | 313.8 (19.9) |
| DMSO | 114.2 (16.7) | 53.5 (5.1)† | 56.7 (17.0)† | 231.4 (22.4) | 144.1 (17.1)† | 162.7 (8.6)† |
| E2 | 146.7 (14.6) | 120.3 (16.3) | 180.5 (5.7) | 266.5 (11.4) | 292.8 (20.6) | 305.3 (27.7) |
| TWEAK- shRNA | 115.3 (10.4) | 33.1 (10.1)* | 48.1 (21.0)* | 235.5 (22.1) | 144.4 (35.0)* | 275.9 (37.5) |
| Control-shRNA | 134.9 (16.8) | 113.9 (12.9) | 172.5 (16.6) | 256.7 (18.4) | 294.6 (40.5) | 298.4 (17.8) |
Data are presented as mean (s.d.).
*P < 0.05 compared with ovariectomized MRL/lpr female mice treated with 17β-estradiol (E2) at the same time.
†P < 0.05 compared with sham-operated MRL/lpr female mice at the same time. Sham: MRL/lpr female mice with sham operation; DMSO: MRL/lpr female mice with ovariectomy treated with dimethyl sulphoxide (DMSO) vehicle alone; E2: MRL/lpr female mice with ovariectomy treated with E2; TWEAK-shRNA: MRL/lpr female mice with ovariectomy treated with E2 plus lentivirus-TNF-like weak inducer of apoptosis–short hairpin RNA (LV-TWEAK-shRNA); control-shRNA: MRL/lpr female mice with ovariectomy treated with E2 plus LV-control-shRNA.
No significant difference was found in serum anti-dsDNA IgG levels between groups at day 8 (Table 2). At day 15 (Table 2), ovariectomized female mice treated with DMSO vehicle had significantly lower serum anti-dsDNA IgG levels [144.1 (17.1) ng/l, P < 0.05] compared with the sham-operated mice [307.1 (12.6) ng/l, P < 0.05]. E2 treatment significantly increased serum anti-dsDNA IgG levels in ovariectomized female mice [292.8 (20.6) ng/l, P < 0.05], and LV-TWEAK-shRNA treatment reversed this effect [144.4 (35.0) ng/l, P < 0.05], but LV-control-shRNA did not affect anti-dsDNA IgG levels [294.6 (40.5) ng/l]. At day 29 (Table 2), compared with DMSO vehicle treatment [162.7 (8.6) ng/l], E2 treatment upregulated the levels of serum anti-dsDNA IgG [305.3 (27.7) ng/l, P < 0.05] in ovariectomized MRL/lpr mice. Neither LV-TWEAK-shRNA nor LV-control-shRNA affected anti-dsDNA IgG levels.
Discussion
TWEAK is a member of the TNF ligand superfamily originally described by Chicheportiche et al. [1] in 1997, and fibroblast growth factor-inducible-14 (Fn14) was identified as its receptor in 2001 [35]. Initial studies focused on a potential role of TWEAK/Fn14 interactions during apoptosis [36], angiogenesis [37, 38], non-immune-mediated inflammation [39] and immune-mediated inflammation [40, 41], especially in LN. However, little is known about its upstream regulatory mechanisms.
SLE is a prototypic inflammatory autoimmune disease, characterized by a significant gender bias. Previous studies have determined a key role of oestrogens in SLE pathogenesis [42]. In this study, we found that TWEAK mRNA expression in PBMCs obtained from LN patients was induced by E2, and it decreased following treatment with ICI 182 780 or MPP. In vivo investigations showed that the expression of renal TWEAK mRNA and protein was significantly reduced in ovariectomized MRL/lpr female mice. E2 administration significantly upregulated renal TWEAK mRNA and protein levels, and this upregulation was inhibited by co-treatment with ICI 182 780 or MPP. Some authors have determined that female patients with acute ST-elevation MI have significantly higher serum levels of sTWEAK on admission, compared with male patients with acute ST-elevation MI [7]. In SLE murine models, female NZB.Yaa mice show increased levels of renal TWEAK mRNA expression, compared with male NZB mice [8–10]. The published data and our results indicate that TWEAK expression may be influenced by female sex hormones. Our results demonstrate for the first time, in vivo and in vitro, that E2 can mediate TWEAK expression, most likely through an ERα-dependent pathway.
Oestrogens/ER/cytokine signalling pathways play very important roles in the pathogenesis of SLE [43]. In intact ERα wild-type mice, E2 treatment induces a lupus phenotype, shortening life span and increasing kidney damage, as well as autoantibody production and the production of multiple Th2-type serum cytokines, including IL-4, IL-5, IL-6, IL-10, INF-γ and TNF-α. Minimal changes are observed in ERα knockout mice following the E2 treatment [26]. In NZB/NZW F1 female mice, ERα knockout alleviates the development of glomerulonephritis, increases survival, and decreases the levels of anti-histone/DNA antibodies and anti-dsDNA IgG antibodies [44]. Here, we showed that renal damage was less severe in ovariectomized female mice treated with DMSO vehicle, compared with the sham-operated mice. Following E2 administration, ovariectomized female mice showed more severe renal damage, while ovariectomized female mice that were treated with E2 combined with LV-TWEAK-shRNA showed a reduction in kidney pathological scores, remission of albuminuria and amelioration of serum creatinine and blood urea nitrogen levels. We suggest that E2 exerts its pathogenic effects in LN partially through the positive modulation of TWEAK expression.
We also demonstrated that LV-TWEAK-shRNA treatment downregulated IL-6 serum levels induced by E2, at days 15 and 29, in the ovariectomized MRL/lpr mice, associated with a significant decrease in TWEAK mRNA expression. IL-6 was demonstrated to be a strong promoter of LN [45]. These results suggest that TWEAK plays an important role in the immune-mediated inflammation, which is consistent with previously reported results. It was reported that TWEAK promotes local expression of multiple inflammatory mediators in human kidney cells [46, 47], including IFN-γ-induced protein of 10 kDa, macrophage inflammatory protein-1α, monocyte chemoattractant protein-1, intercellular cell adhesion molecule-1, vascular cell adhesion molecule-1 and regulated upon activation normal T cell expressed and secreted protein [48].
It is believed that autoantibodies, including anti-dsDNA IgG, are deposited in the kidneys, which induces renal diseases. Zhao et al. [8] reported that in the chronic graft-vs-host model of SLE, anti-TWEAK antibody treatment decreases kidney IgG deposition, but has no effect on the serum levels of autoantibodies, at all time points. Furthermore, the knockout of TWEAK receptor Fn14 significantly improves glomerulonephritis in lupus mice, but does not significantly alter systemic autoantibody levels [8, 33]. In our study, TWEAK-shRNA treatment led to a decrease in the serum levels of anti-dsDNA IgG induced by E2, at day 15. However, TWEAK-shRNA treatment did not inhibit the increase in the serum anti-dsDNA IgG levels induced by E2 at day 29, which is consistent with the previous observations. The discrepancy of results obtained in these studies may be due to the different murine models and interventions. There is evidence that oestrogens influence SLE progression, as demonstrated by the study in which E2-treated B/W mice had significantly higher serum total IgG concentration and anti-DNA antibody (IgG2a and IgG3 subclasses) levels compared with vehicle controls [23]. These results may suggest that TWEAK can temporally influence the changes in dsDNA antibody expression levels effected by oestrogen. Future studies should be conducted to address the biological mechanism for this effect.
Taken together, the results obtained in this study showed that TWEAK could be regulated by E2, most likely through the ERα pathway, and that it was associated with kidney damage in MRL/lpr mice. LV-TWEAK is an effective therapeutic agent in the treatment of LN. However, the exact mechanisms of E2/ERα signalling pathway–mediated modulation of TWEAK expression remain unknown, and further investigations are required in order to determine these mechanisms.
Acknowledgements
We thank Dr Zhao Haifeng (Department of Pathology, the Second Affiliated Hospital of Soochow University) for his help with histopathological sections, and Dr Sun Lingyun (Central Laboratory, the Second Affiliated Hospital of Soochow University) for assistance with image analysis.
Funding: This work was partially supported by the Suzhou Science and Technology Development Project (Grant No. SYS201231) and the National Natural Science Foundation of China (Grant No. 81200507).
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
Author notes
*Leixi Xueand ZhiQin Liu contributed equally to this study




Comments