-
PDF
- Split View
-
Views
-
Cite
Cite
N. J. Minaur, C. Jefferiss, A. K. Bhalla, J. N. Beresford, Methotrexate in the treatment of rheumatoid arthritis. I. In vitro effects on cells of the osteoblast lineage, Rheumatology, Volume 41, Issue 7, July 2002, Pages 735–740, https://doi.org/10.1093/rheumatology/41.7.735
Close - Share Icon Share
Abstract
Objective. Low‐dose methotrexate (MTX) is often used in the treatment of rheumatoid arthritis (RA). To be effective, treatment must be long‐term, and there are concerns that MTX may impair bone formation in a population already predisposed to osteoporosis. The purpose of this investigation was to determine the direct effects of MTX at clinically relevant doses on the growth and differentiation of human cells of the osteoblast (bone‐forming) lineage.
Methods. Cells derived from the marrow stroma (BMSC) and trabecular surfaces [human bone‐derived cells (HBDC)] of adult ribs were cultured in the absence or presence of MTX (1–1000 nM). To promote the differentiation and further maturation of cells of the osteoblast lineage, one half of the cultures were treated additionally with 10 nM dexamethasone (Dx).
Results. In cultures of BMSC, treatment with MTX (±Dx) did not affect the total number of colonies that formed or the expression of the developmental markers STRO‐1 and alkaline phosphatase (AP). At concentrations ≥10 nM, however, there was a statistically significant reduction in the number of cells harvested at the end of primary culture. In cultures of HBDC, treatment with MTX (in the presence of Dx) did not affect cell number or the expression of AP.
Conclusions. At concentrations ≥10 nM, treatment with MTX inhibits the proliferation of primitive marrow stromal cells, but not their ability to undergo osteogenic differentiation. The proliferation and further maturation of cells of the osteoblast lineage is not affected by treatment with MTX. These findings are reassuring for clinicians using MTX in the treatment of RA.
The drug methotrexate (MTX) is used in high doses (100–1000 mg/m2) to treat malignancies [1] and in low doses (5–25 mg/week) to treat rheumatoid arthritis (RA) and other inflammatory conditions [2]. The main action of MTX is inhibition of dihydrofolate reductase, which reduces dietary folate to tetrahydrofolate, an essential cofactor in DNA and RNA synthesis, although MTX also has many other effects on cytokines and the inflammatory response [3]. High‐dose MTX impairs bone formation, particularly in children [4, 5], and there are case reports of fragility fractures in adults on long‐term, low‐dose MTX therapy for conditions such as RA and psoriatic arthritis [6–8]. In vivo studies have used high‐dose and low‐dose MTX, both in normal animals and in animal models of arthritis. Low‐dose MTX (corresponding to the doses used to treat RA in humans), when given to normal rats, suppressed osteoblast function [9]. However, when given to rats with adjuvant‐induced arthritis, bone mass was maintained in the MTX‐treated animals compared with controls (which also had adjuvant arthritis) [10]. In vitro, MTX has been reported to inhibit the proliferation of cells of the osteoblast lineage but to have no effect on their differentiation [11, 12]. As RA predisposes patients to osteoporosis, it would be undesirable to use a drug as therapy which may adversely affect bone formation and therefore increase the risk of fragility fractures.
In the present study, the effects of MTX on parameters of growth and differentiation in cultures of human bone‐derived cells (HBDC) were investigated. Cultures derived from the marrow stroma [bone marrow stromal cells (BMSC)] were used to assess the effects of the drug on the colony‐forming potential and osteogenic differentiation of the primitive mesenchymal precursors [colony‐forming units, fibroblastic (CFU‐F)] that are a source of bone‐forming cells in the adult skeleton [reviewed in 13]. Cultures derived from explants of trabecular bone were used to assess the effects of MTX on the proliferation and further maturation of cells of the osteoblast lineage (HBDC). Experiments were conducted on cells cultured in the absence and presence of the synthetic glucocorticoid dexamethasone (Dx; 10 nM), as this has been shown to promote the differentiation and further maturation of cells of the osteoblast lineage in both culture types [14–16]. On the basis of the findings of Bologna et al. [17], MTX was used at 1–1000 nM. These authors assayed the concentrations of MTX in the synovial membrane and cortical and trabecular bone of 10 RA patients treated with the drug for a mean duration of 26 months. They were able to do this because the patients were undergoing surgery; five were having wrist synovectomy, two each having shoulder and knee arthroplasty and one having a hip arthroplasty. The day prior to surgery, each patient had an intramuscular injection of 10 mg MTX. They were found to have similar high mean levels of MTX in the synovial membrane (0.285 nmol/g) and in both trabecular (0.292 nmol/g) and cortical (0.286 nmol/g) bone, while the plasma concentration was 10‐fold less (0.0252 nmol/ml).
Materials and methods
Cell culture
The methods used for the isolation, culture and subculture of BMSC and HBDC have been described in detail elsewhere and were followed without further modification [14, 15]. For both cell types the starting material was segments of rib from six patients undergoing thoracotomy: three were males, age range 19–83 yr, and four had a malignancy (lung or oesophageal cancer). The other two diagnoses were recurrent pneumothorax and diaphragmatic hernia. Consent was given by subjects for use of the tissue. No patients had arthritis and one woman was on hormone replacement therapy, but none were on other medication known to affect bone.
Colony formation
After 21 days, colonies were washed with phosphate‐buffered saline (Ca2+‐ and Mg2+‐free), pH 7.4, fixed for 5 min at room temperature and then allowed to air‐dry. Colonies were stained with fast red and then methylene blue, and the number of colonies formed containing ⩾50 cells and the proportion expressing alkaline phosphatase (AP) were determined as previously described [18] using an Olympus SZ‐CTV microscope (×15 objective).
Cell number
The number of BMSC was determined as described previously [16], using an electronic particle counter (Coulter Electronics, Luton, UK). The number of HBDC was determined indirectly using an assay based on the binding of methylene blue stain to the fixed cell layer [11, 19]. We [16, 20] and others [21] have reported previously that cells derived from different donors exhibit marked variation in their capacity for proliferation. For this reason, and for the sake of clarity, the proliferation data are presented as a percentage of control.
Expression of the developmental markers STRO‐1 and AP
STRO‐1 is a trypsin‐resistant, cell‐surface antigen expressed by a subset of human marrow stromal cells that includes essentially all assayable CFU‐F [22]. We [23] and others [24] have shown that a monoclonal antibody recognizing this antigen can be used in combination with one recognizing the bone/live/kidney isozyme of AP (B4‐78 [25]) to identify cells of the osteoblast lineage at different stages of maturation. The monoclonal antibody STRO‐1 (IgM subclass) was obtained from Dr Paul Simmons (Peter MacCallum Cancer Institute, Melbourne, Australia) and B4‐78 (IgG1 subclass) from the Developmental Studies Hybridoma Bank (University of Iowa, IA, USA). The expression of these antigens was monitored by flow cytometry (FACSStarPlus; Becton Dickinson) as described previously and without further modification [16, 23].
Statistics
Experiments were routinely set up with quadruplicates of each condition. Results were analysed with the paired t‐test or analysis of variance (ANOVA) with Fisher's protected least significant difference (PLSD), and the level of significance was set at 95%.
Results
Colony‐forming efficiency
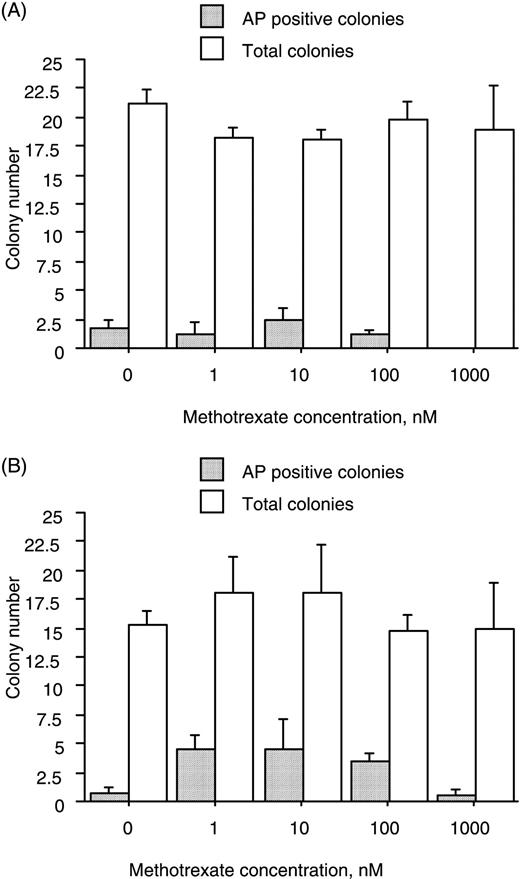
Treatment with MTX (1–1000 nM), irrespective of the presence or absence of Dx, had no consistent effect on the total number of colonies that formed or on the proportion that expressed the developmental marker AP (Fig. 1).
Culture in the presence of MTX does not affect the colony‐forming efficiency of marrow cell suspensions. Cells were cultured for 18 days in the absence or presence of MTX at the concentrations indicated. MTX had no consistent effect on the total number of colonies that formed or on the proportion that were positive for AP either in the absence (A) or presence (B) of 10 nM Dx. Data (mean and s.e.m.) represent three donors.
Proliferation of BMSC
At concentrations ⩾10 nM, treatment with MTX was associated with a statistically significant decrease in the number of cells harvested at the end of secondary culture. The magnitude of the observed decrease was similar in control and Dx‐treated cultures (Fig. 2).
Treatment with MTX decreases cell number in cultures of human marrow stromal cells. BMSC were cultured for 37–66 days in the absence or presence of MTX at the concentrations indicated. Treatment with MTX at 10 nM caused a significant reduction in cell number (P<0.05, Student's t‐test). The magnitude of the MTX‐induced decrease in cell number was similar in control and Dx‐treated cultures. Data are percentages of control cell numbers (mean and s.e.m.) and represent two donors.
Expression of the developmental markers STRO‐1 and AP
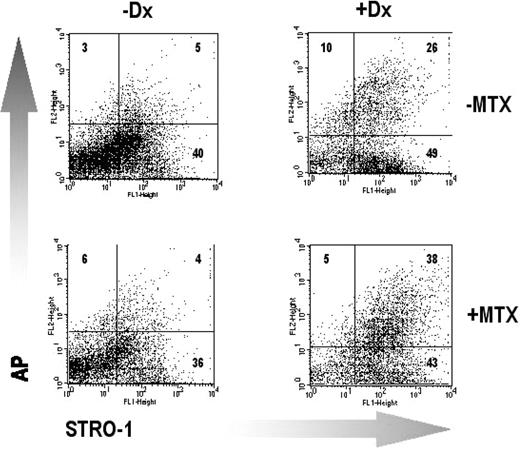
In cultures derived from three donors, treatment in primary and secondary culture [total of 71±3 days (mean±s.e.m.)] with 100 nM MTX had no consistent effect on the proportion of cells that expressed STRO‐1, AP or both markers in combination (Fig. 3 and Table 1).
Treatment with MTX does not affect the expression of the developmental markers STRO‐1 and AP in BMSC cultured in the absence or presence of Dx. The results are shown for a single representative donor. The number of cells (percentage of total) recovered in each quadrant is shown. Further details are given in the legend to Table 1.
Lack of effect of MTX on the expression of the developmental markers STRO‐1 and AP by BMSC cultured in the absence or presence of Dx
| % Positive cells (mean±s.e.m.) | ||||
| Surface phenotype | Dexamethasone | Control | Methotrexate | |
| STRO‐1−/AP− | − | 36±8 | 21±4 | |
| + | 15±6 | 32±12 | ||
| STRO‐1+/AP− | − | 47±7 | 48±3 | |
| + | 32±11 | 32±9 | ||
| STRO‐1+/AP+ | − | 12±1 | 27±8 | |
| + | 41±12 | 24±8 | ||
| STRO‐1−/AP+ | − | 5±1 | 13±9 | |
| + | 4±1 | 12±6 | ||
| % Positive cells (mean±s.e.m.) | ||||
| Surface phenotype | Dexamethasone | Control | Methotrexate | |
| STRO‐1−/AP− | − | 36±8 | 21±4 | |
| + | 15±6 | 32±12 | ||
| STRO‐1+/AP− | − | 47±7 | 48±3 | |
| + | 32±11 | 32±9 | ||
| STRO‐1+/AP+ | − | 12±1 | 27±8 | |
| + | 41±12 | 24±8 | ||
| STRO‐1−/AP+ | − | 5±1 | 13±9 | |
| + | 4±1 | 12±6 | ||
BMSC derived from multiple donors (n=3) were cultured (±10 nM Dx) for a total of 71±3 days (mean±s.e.m.) in the absence or presence of 100 nM MTX. At the end of this period the cells were harvested and the proportions of cells expressing the developmental markers STRO‐1 and AP were determined by flow cytometry. Treatment with 100 nM MTX had no significant effect on the proportion of cells recovered in the different quadrants either in the absence or presence of Dx (ANOVA with Fisher's PLSD post hoc testing).
Lack of effect of MTX on the expression of the developmental markers STRO‐1 and AP by BMSC cultured in the absence or presence of Dx
| % Positive cells (mean±s.e.m.) | ||||
| Surface phenotype | Dexamethasone | Control | Methotrexate | |
| STRO‐1−/AP− | − | 36±8 | 21±4 | |
| + | 15±6 | 32±12 | ||
| STRO‐1+/AP− | − | 47±7 | 48±3 | |
| + | 32±11 | 32±9 | ||
| STRO‐1+/AP+ | − | 12±1 | 27±8 | |
| + | 41±12 | 24±8 | ||
| STRO‐1−/AP+ | − | 5±1 | 13±9 | |
| + | 4±1 | 12±6 | ||
| % Positive cells (mean±s.e.m.) | ||||
| Surface phenotype | Dexamethasone | Control | Methotrexate | |
| STRO‐1−/AP− | − | 36±8 | 21±4 | |
| + | 15±6 | 32±12 | ||
| STRO‐1+/AP− | − | 47±7 | 48±3 | |
| + | 32±11 | 32±9 | ||
| STRO‐1+/AP+ | − | 12±1 | 27±8 | |
| + | 41±12 | 24±8 | ||
| STRO‐1−/AP+ | − | 5±1 | 13±9 | |
| + | 4±1 | 12±6 | ||
BMSC derived from multiple donors (n=3) were cultured (±10 nM Dx) for a total of 71±3 days (mean±s.e.m.) in the absence or presence of 100 nM MTX. At the end of this period the cells were harvested and the proportions of cells expressing the developmental markers STRO‐1 and AP were determined by flow cytometry. Treatment with 100 nM MTX had no significant effect on the proportion of cells recovered in the different quadrants either in the absence or presence of Dx (ANOVA with Fisher's PLSD post hoc testing).
Proliferation of HBDC
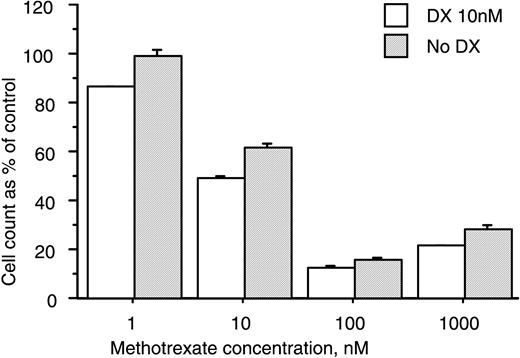
Treatment with MTX (1–1000 nM) did not affect the proliferation of HBDC whether cultured in the absence or presence of 10 nM Dx (Fig. 4).
Treatment with MTX does not affect the proliferation of HBDC. HBDC were cultured with or without 10 nM Dx for a total of 8 days in the absence or presence of MTX at the concentrations indicated. Cell number was assessed indirectly using the methylene blue binding assay [19]. Data are percentages of the optical density at 650 nm in control cultures (mean and s.e.m.), and represent two donors.
HBDC expression of the developmental marker AP
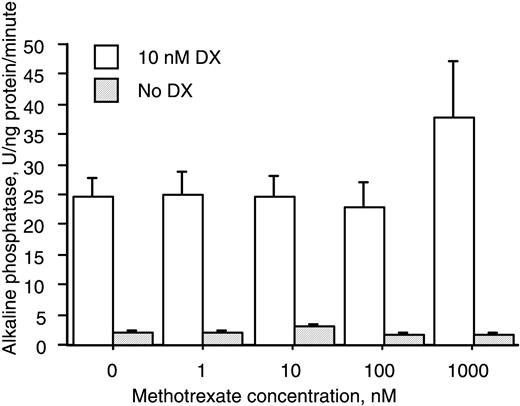
Treatment of HBDC with MTX (1–1000 nM) did not affect either basal or Dx‐stimulated expression of AP (Fig. 5).
Treatment with MTX does not affect the expression of AP in cultures of HBDC. HBDC were cultured with or without 10 nM Dx for a total of 8 days in the absence or presence of MTX at the indicated concentrations. Data (mean and s.e.m.) represent two donors.
Discussion
Concerns have been expressed that low‐dose MTX, as used in the treatment of RA, might have deleterious effects on bone formation. This prompted us to investigate the direct effects of this disease‐modifying anti‐rheumatic drug on human cells of the osteoblast lineage in vitro. In adults, bone cells of the osteoblast lineage are derived from primitive mesenchymal precursors (CFU‐F) associated with the soft, fibrous tissue of the marrow stroma and bone surfaces. Our results show that treatment with MTX, at concentrations similar to those measured in the bones of RA patients [17], has no consistent effect on the ability of these cells to assume an adherent phenotype and form colonies when explanted in vitro. In addition, they show that treatment with MTX has no consistent effect on their ability to give rise to cells of the osteoblast lineage (no effect on the expression of STRO‐1 and/or AP) under basal conditions or when stimulated with 10 nM Dx. They do, however, indicate that the proliferative potential of these primitive precursors and/or their immediate progeny may be affected adversely by treatment with MTX at concentrations ≥10 nM.
In contrast to the results obtained in cultures of BMSC, no consistent effect of MTX was observed on proliferation in cultures of HBDC. These cells have been characterized extensively and, when compared with BMSC, shown to express a more mature osteoblast‐like phenotype [14–15]. This suggests that the inhibitory effects of MTX on proliferation may be restricted to less mature cells of the marrow stromal hierarchy, as has been shown recently by Uehara et al. [26] using cultures derived from murine bone and marrow. Consistent with this possibility, although we found no effect of MTX on the proliferation of HBDC in secondary culture, we did observe that its inclusion in primary culture delayed the appearance of cells on the surface of and migration from the trabecular explants (data not shown).
HBDC can be induced to undergo further osteogenic maturation by culture in the presence of a physiologically equivalent amount of glucocorticoid [14, 15], in this case 10 nM Dx. Our results show that this potential for further maturation is not compromised by culture in the presence of MTX, at least when assessed on the basis of increased expression of AP.
In an earlier study, and in contrast to the results presented here, Scheven et al. [11] found that the proliferation of HBDC, assessed, as in this study, using an assay based on the binding of methylene blue, was inhibited by MTX at concentrations ⩾10 nM. The reason(s) for this discrepancy in our findings is not entirely clear. One possibility is the use of cells from donors with different underlying pathology and/or from different skeletal sites (ribs vs head of femur). Another is our use of improved conditions of culture, particularly with regard to the maintenance of adequate ascorbate nutrition, which have been shown to enhance the proliferation and osteogenic differentiation of HBDC [14, 15].
The results of in vivo investigations suggest that low‐dose MTX, as used in the treatment of RA, does not adversely affect histomorphometric or biochemical (urinary deoxypyrodinoline) indices of bone resorption [27, 28]. To our knowledge, the direct effects of MTX on human cells of the osteoclast lineage have not been investigated; this reflects the difficulty in isolating cells of this type in a number and purity sufficient for further investigation, compared with cells of the osteoblast lineage. Recently, however, considerable progress has been made in this regard [29–32], and it is to be hoped that this will lead to improved understanding of the direct effects of MTX on human osteoclasts and their immediate precursors.
In conclusion, we have shown that treatment with MTX can inhibit the proliferation of CFU‐F and their immediate progeny, but that it does not appear to exert a direct inhibitory influence on the proliferation and/or differentiation of cells of the osteoblast lineage. From a clinical perspective this result is reassuring, as it provides additional evidence to support the hypothesis that long‐term use of MTX in the treatment of RA is unlikely to have a major impact on bone formation. In a prospective study of 116 patients with RA this proved to be the case [28].
Correspondence to: N. J. Minaur, 66 Third Avenue, Bath BA2 3NZ, UK.
We are grateful to the patients, surgeons and theatre staff at Frenchay Hospital, Bristol, UK, without whom this work would not have been possible, and J. Screen, who gave technical assistance. NJM was supported by a Clinical Research Fellowship (M0530) funded by the Arthritis Research Campaign, UK.
References
Jolivet J, Cowan KH, Curt GA, Clendeninn NJ, Chabner BA. The pharmacology and clinical uses of methotrexate.
Constantin A, Loubet‐lescoulié P, Lambert N et al. Antiinflammatory and immunoregulatory action of methotrexate in the treatment of rheumatoid arthritis.
Ecklund K, Laor T, Goorin AM, Connolly LP, Jaramillo D. Methotrexate osteopathy in patients with osteosarcoma.
Sambrook PN. Osteoporosis in rheumatoid arthritis: what is the role of antirheumatic therapy?
Schapira D, Scharf Y. Insufficiency fracture of the distal tibia mimicking arthritis in a rheumatoid arthritis patient. The possible role of methotrexate treatment.
Maenaut K, Westhovens R, Dequeker J. Methotrexate osteopathy: does it exist?
May KP, West SG, McDermott MT, Huffer WE. The effect of low‐dose methotrexate on bone metabolism and histomorphometry in rats.
Segawa Y, Yamaura M, Aota S et al. Methotrexate maintains bone mass by preventing both a decrease in bone formation and an increase in bone resorption in adjuvant‐induced arthritic rats.
Scheven BAA, van der Veen MJ, Damen CA et al. Effects of methotrexate on human osteoblasts in vitro: modulation by 1,25‐dihydroxyvitamin D3.
van der Veen MJ, Scheven BAA, van Roy JLAM, Damen CA, Lafeber FPJG, Bijlsma JWJ. In vitro effects of methotrexate on human articular cartilage and bone‐derived osteoblasts.
Beresford JN. Osteogenic stem cells and the stromal system of bone and marrow.
Gallagher JA, Gundle R, Beresford JN. Isolation and culture of bone‐forming cells (osteoblasts) from human bone. In: Jones GE, ed.
Gundle R, Stewart K, Screen J, Beresford JN. Isolation and culture of human bone‐derived cells. In: Beresford JN, Owen ME.
Walsh S, Jordan GR, Jefferiss CM, Stewart K, Beresford JN. High concentrations of dexamethasone suppress the proliferation but not the differentiation or further maturation of human osteoblast precursors in vitro: relevance to glucocorticoid‐induced osteoporosis.
Bologna C, Edno L, Anaya J‐M et al. Methotrexate concentrations in synovial membrane and trabecular and cortical bone in rheumatoid arthritis.
Scutt A, Bertram P. Basic fibroblast growth factor in the presence of dexamethasone stimulates colony formation, expansion, and osteoblastic differentiation by rat bone marrow stromal cells.
Oliver MH, Harrison NK, Bishop JE, Cole PJ, Laurent GJ. A rapid and convenient assay for counting cells cultured in microwell plates—application for assessment of growth factors.
Walsh S, Jefferiss C, Stewart K, Jordan GR, Screen J, Beresford JN. Expression of the developmental markers STRO‐1 and alkaline phosphatase in cultures of human marrow stromal cells: Regulation by fibroblast growth factor (FGF‐2) and relationship to the expression of FGF receptors 1–4.
Phinney DG, Kopen G, Righter W, Webster S, Tremain N, Prockop DJ. Donor variation in the growth properties and osteogenic potential of human marrow stromal cells.
Simmons PJ, Torok‐Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO‐1.
Stewart K, Walsh S, Screen J et al. Further characterisation of cells expressing STRO‐1 in cultures of adult human bone marrow stromal cells.
Gronthos S, Zannettino ACW, Graves SE, Ohta S, Hay SJ, Simms PJ. Differential cell surface expression of the stro‐1 and alkaline phosphatase antigens on discrete developmental stages in primary cultures of human bone cells.
Lawson GM, Katzmann JA, Kimlinger TK, O'Brien JF. Isolation and preliminary characterization of a monoclonal antibody that interacts preferentially with the liver isoenzyme of human alkaline phosphatase.
Uehara R, Suzuki Y, Ichikawa Y. Methotrexate (MTX) inhibits osteoblastic differentiation in vitro: Possible mechanism of MTX osteopathy.
El Miedany YM, Abubakr IH, El Baddini M. Effect of low dose methotrexate on markers of bone metabolism in patients with rheumatoid arthritis.
Minaur NJ, Kounali D, Vedi S, Compston JE, Beresford JN, Bhalla AK. Methotrexate in the treatment of rheumatiod arthritis. II. In vivo effects on bone mineral density.
Fujikawa Y, Sabokbar A, Neale S, Athanasou NA. Human osteoclast formation and bone‐resorption by monocytes and synovial macrophages in rheumatoid arthritis.
Itonaga I, Fujikawa Y, Sabokbar A, Murray DW, Athanasou NA. Rheumatoid arthritis synovial macrophage–osteoclast differentiation is osteoprotegerin ligand‐dependent.
Massey HM, Flanagan AM. Human osteoclasts derive from CD14‐positive monocytes.



![Treatment with MTX does not affect the proliferation of HBDC. HBDC were cultured with or without 10 nM Dx for a total of 8 days in the absence or presence of MTX at the concentrations indicated. Cell number was assessed indirectly using the methylene blue binding assay [19]. Data are percentages of the optical density at 650 nm in control cultures (mean and s.e.m.), and represent two donors.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/rheumatology/41/7/10.1093/rheumatology/41.7.735/2/m_RHE12220-4.jpeg?Expires=1716480917&Signature=zlmFBjURh5T5ZBxiZFY-FdknAAe3DKeDa0tlib~fm128kmm2gREokyXRUy0i-nUs9oDCtWXu0Bnva9X4fYxX7JewBjqLU117e6DDDpV6VXYRhyHZZt3I07oo-ec9vH~cyQ1mbsRbs8tsx~g~bwwgvSMJ2DDXvgCSxOb0IAQa0p7XsCqwH65b0~00lygyC9hvE1ArDUAoReeUDgeYKFA-FByNhZgVqeJ3w4HJPG0kJgP-wIiy88i503jTEfmcCx3zxX5Vu~HU3EmEO1J~LINVkJFkW2WVXvFaXoUVreS-6OHw1D--fn~uBri-1LPiwIdJcDnGqVVJQ-mLmiDlys-LSQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

Comments